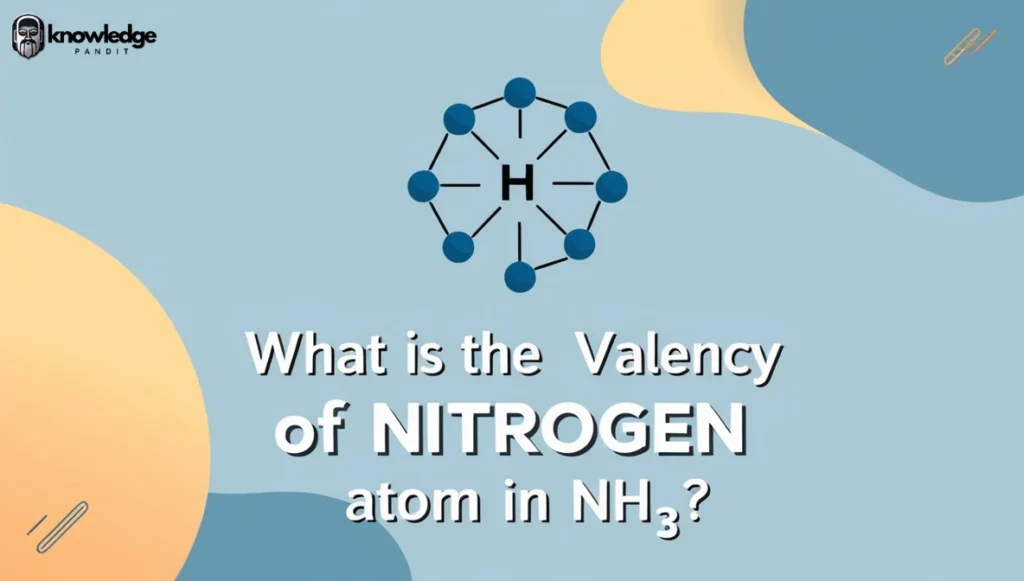
Answer: The Valency of Nitrogen Atom in NH₃ is 3
Nitrogen is a non-metal chemical element with the symbol N and atomic number 7. It constitutes about 78% of Earth’s atmosphere and is an essential element for life.
Electronic Configuration of Nitrogen
The electronic configuration of nitrogen is:
- N (Z=7): 1s² 2s² 2p³
- In shorthand notation: [He] 2s² 2p³
Nitrogen has 5 valence electrons in its outermost shell (2s² 2p³).
Why valency of Nitrogen in NH₃ is 3?
- To achieve a stable octet, nitrogen needs 3 additional electrons, which it obtains by forming three covalent bonds with three hydrogen atoms in ammonia (NH₃).
- Thus, the valency of nitrogen in NH₃ is 3.
Explore More:
- What is Spectrum and What are its Types?
- What is the Molecular Mass of Nitrogen?
- How many Electrons are there in One-Coulomb Charge?
- What Chemical Process Is Used for Obtaining a Metal from Its Oxide?
- Why is force said to be the rate of change of momentum and not just change
- in momentum?
Key Points to Remember
- Valency: The combining capacity of an atom, determined by the number of electrons it can share, lose, or gain to achieve stability.
- In NH₃, nitrogen shares its electrons through covalent bonding.
This property of nitrogen makes it a crucial component in many organic and inorganic compounds.