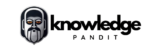Electrolytic refining is a widely used process for purifying metals. The principle involves the use of electricity to separate pure metal from its impure form.
Principle of Electrolytic Refining
The method operates on the principle that when an electric current flows through an electrolyte, metal ions migrate from the anode to the cathode, resulting in the deposition of pure metal at the cathode. Impurities accumulate as a by-product, commonly referred to as anode sludge.
Key Components of the Process:
- Anode: The impure metal to be refined.
- Cathode: A strip of pure metal where the refined metal is deposited.
- Electrolyte: A solution containing a salt of the metal being refined, which facilitates ion exchange.
Steps in Electrolytic Refining
- Preparation of Electrolytic Cell:
- The anode is made of impure metal.
- The cathode is a thin strip of pure metal.
- The electrolyte is a solution of the metal’s salt (e.g., copper sulfate for refining copper).
- Passing Electric Current:
- When electricity is passed, metal ions from the anode dissolve into the electrolyte and move towards the cathode.
- These ions are reduced at the cathode, forming a layer of pure metal.
- Collection of Impurities:
- Non-metallic impurities either remain dissolved in the solution or collect at the bottom as anode mud.
Example of Electrolytic Refining
Refining of Copper:
- Anode: Impure copper.
- Cathode: Pure copper strip.
- Electrolyte: Acidified copper sulfate (CuSO₄ + H₂SO₄).
During the process:
- Copper ions (Cu²⁺) from the anode dissolve into the solution.
- These ions migrate and deposit as pure copper at the cathode.
- Impurities like silver and gold settle as anode mud.
Reaction:
At Anode:
Cu → Cu²⁺ + 2e⁻
At Cathode:
Cu²⁺ + 2e⁻ → Cu
Importance of Electrolytic Refining
- High Purity Metals: Produces metals with up to 99.99% purity, essential for industrial and electronic applications.
- Recovery of Valuable Impurities: Precious metals like gold and silver are recovered from the anode mud.
- Wide Application: Used in refining copper, zinc, aluminium, and more.
Explore More:
- What is Splitting of Light?
- What is Succus Entericus?
- What is Pisciculture?
- Is Stone Rigid or Non-Rigid?
Interesting Facts About Electrolytic Refining
- Green Technology: The process is environmentally friendly compared to traditional refining methods.
- Valuable By-products: Electrolytic refining not only purifies metals but also helps recover valuable metals from the impurities.
Electrolytic refining exemplifies the innovative use of electricity in achieving high-purity metals essential for modern industries.